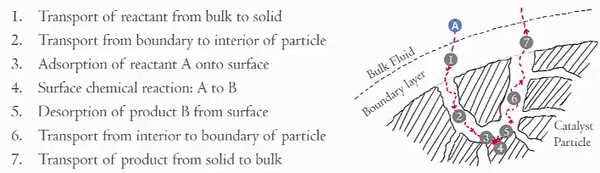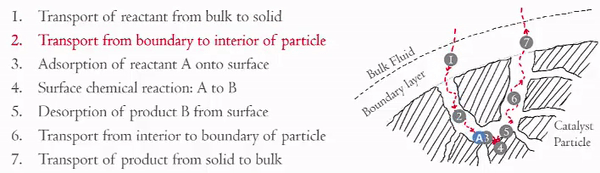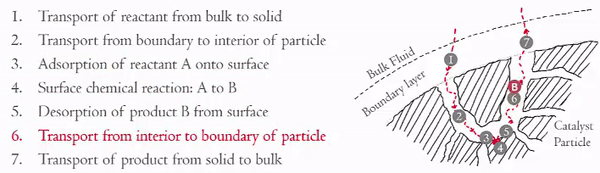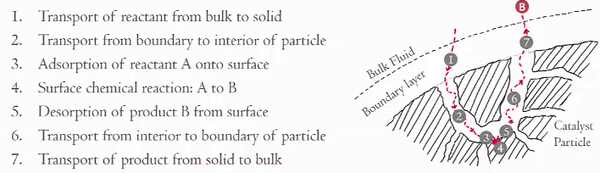
Heterogeneous catalytic reactions can be though of as having seven steps:

The material below examines the external diffusion (Steps 1 and 7).
The external mass transfer is determined by the boundary layer around the object. The video below reviews the derivation of a molar balance through a unit zone and then shows how we can use this to calculate the mass transfer rate across the boundary layer
From the video it is clear that the key parameter for the mass transfer is the mass transfer coefficient, kc, in m s-1. This is dependent on the diffusion coefficient and the boundary layer thickness, and thus is complex to calculate. This means that typically correlations have been created for different situations to allow us to find values of the mass transfer coefficient.
Now that we have an expression for the mass transfer through the boundary layer, we can examine its effect on the overall reaction rate. The video below derives the overall reaction rate for a non-porous catalyst particle with a first order surface reaction:
The overall reaction rate is dependent on the surface reaction rate constant and the mass transfer constant. The graph below gives an example of the variation of the total surface reaction rate (for a first order reaction) with the superficial fluid velocity, U, for a packed bed of spherical particles. The reaction rate constant, k1'', and the particle diameter, dp, can be varied using the sliders. At low velocity the total reaction rate is controlled by the rate of the mass transfer while at high velocity it is controlled by the rate of the reaction.
The internal diffusion (Steps 2 and 6) can be seen under Thiele Modulus.